Entec 0.5/1
- ENG
- မြန်မာ
COMPOSITION
ZIFAM ENTEC 0.5 – Each Film -Coated Tablet Contains: Entecavir (monohydrate) USP equivalent to Entecavir 0.5 mg
ZIFAM ENTEC 1 – Each Film-Coated Tablet Contains: Entecavir (monohydrate) USP equivalent to Entecavir 1 mg
PHARMACOLOGICAL PROPERTIES:
Pharmacotherapeutic category: Antivirals for systemic use, nucleoside and nucleotide reverse transcriptase inhibitors.
ATC code: J05AF10
Mechanism of action
Entecavir, a guanosine nucleoside analogue with activity against HBV polymerase, is efficiently phosphorylated to the active triphosphate TP) form, which has an intracellular half-life of 15 hours. By competing with the natural substrate deoxyguanosine TP, entecavir-TP functionally inhibits the 3 activities of the viral polymerase: (1) priming of the HBV polymerase, (2) reverse transcription of the negative strand DNA from the pregenomic messenger RNA, and (3) synthesis of the positive strand -BV DNA. The entecavit-TP Ki for HBV DNA polymerase is 0 0012 μm.
Entecavi-TP is a weak inhibitor of cellular DNA polymerases α,β and δ with Ki values of 18 to 40 μm. In addition, high exposures of entecavir had no relevant adverse effects on y polymerase or mitochondrial DNA synthesis in HepG2 cells (Ki > 160 μm).
PHARMACOKINETIC PROPERTIES
Absorption: Entecavir is rapidly absorbed with peak plasma concentrations occurring between 0 5-1,5 hours. The absolute bioavailability has not been determined. Based on urinary excretion of unchanged drug, the bioavailability has been estimated to be at least 70%. There is a dose-proportionate increase in Cmax and AUC values following multiple doses ranging from 0.1-1 mg. steady-state is achieved between 6-10 days after once daily dosing with ≈ 2 times accumulation. Cmax and Cmin at steady-state are 4.2 and 0.3 ng/mL, respectively, for a dose of 0.5 mg, and 8 2 and 0.5 ng/mL, respectively, for 1 mg. The tablet and oral solution were bioequivalent in healthy subjects; therefore, both forms may be used interchangeably.
Administration of 0.5 mg entecavir with a standard high-fat meal (945 kcal, 54.6 g fat) or a light meal (379 kcal, 8.2 g fat) resulted in a minimal delay in absorption (1-1,5 hour fed vs. 0.75 hour lasted), a decrease in Cmax of 44-46%, and a decrease in AUC of 18-20%. The lower Cmax and AUC when taken with food is not considered to be of clinical relevance in nucleoside-naive patients but could affect efficacy in lamivudine-refractory patients.
Distribution: The estimated volume of distribution for entecavir is in excess of total body water, Protein binding to human serum protein in vitro is ≈ 13%.
Biotransformation: Entecavir is not a substrate, inhibitor or inducer of the CYP450 enzyme system. Following administration of 14C-entecavir, no oxidative or acetylated metabolites and minor amounts of the phase Il metabolites, glucuronide and sulfate conjugates, were observed.
Elimination: Entecavir is predominantly eliminated by the kidney with the urinary recovery of unchanged drug at steady-state of about 75% of the dose. Renal clearance is independent of dose and ranges between 360-471 mL/min suggesting that entecavir undergoes both glomerular filtration and net subular secretion. After reaching peak levels, entecavir plasma concentrations decreased in a bi-exponential manner with a terminal elimination half-life of ≈ 128-149 hours. The observed drug accumulation index is ≈ 2 times with once-daily dosing, suggesting an effective accumulation half-life of about 24 hours.
THERAPEUTIC INDICATIONS
Zifam Entec is indicated for the treatment of chronic hepatitis B virus (HBV) infection in adults with:
• compensated liver disease and evidence of active viral replication, persistently elevated serum alanine aminotransferase (ALT) levels and histological evidence of active inflammation and/or fibrosis.
• decompensated liver disease.
For both compensated and decompensated liver disease, this indication is based on clinical data in nucleoside naive patients with HBeAg positive and HBeAg negative HBV infection. With respect to patients with lamivudine-refractory hepatitis B.
Entecavir is also indicated for the treatment of chronic HBV Infection in nucleoside naive paediatric patients from 2 to < 18 years of age with compensated liver disease who have evidence of active viral replication and persistently elevated serum ALT levels, or histological evidence of moderate to severe inflammation and/or fibrosis. With respect to the decision to initiate treatment in paediatric patients.
POSOLOGY AND METHOD OF ADMINISTRATION
Therapy should be initiated by a physician experienced in the management of chronic hepatitis B infection.
Posology Compensated liver disease
Nucleaside naive patients: The recommended dose in adults is 0.5 mg once daily, with or without food.
Lamivudine-refractory patients (i.e. with evidence of viraemia white on lamivudine or the presence of lamivudine resistance [LVDr] mutations): the recommended dose in adults is 1 mg once daily, which must be taken on an empty stomach (more than 2 hours before and more then 2 hours after a meal), In the presence of LVDr mutations, combination use of entecavir plus a second antiviral agent (which does not share cross-resistance with either lamivudine or entecavir should be considered in preference to entecavir monotherapy.
Decompensated liver disease
The recommended dose for adult patients with decompensated liver disease is 1 mg once daily, which must be taken on an empty stomach (more than 2 hours before and more than 2 hours after a meal).
In patients with decompensated liver disease or cirrhosis, treatment cessation is not recommended.
Paediatric population
Paediatric patients with body weight of at least 32.6 kg, should be administered a daily dose of one 0.5 mg tablet with or without food.
• In HBeAg positive paediatric patients, treatment should be administered for at least 12 months after achieving undetectable HBV DNA and HBeAg seroconversion (HBeAg loss and anti-HBe detection on two consecutive serum samples at least 3-6 months apart) or until HBs seroconversion or there is loss of efficacy. Serum ALT and HBV DNA levels should be followed regularly after treatment discontinuation
• In HBeAg negative paediatric patients, treatment should be administered until HBs seroconversion or there is evidence of loss of efficacy.
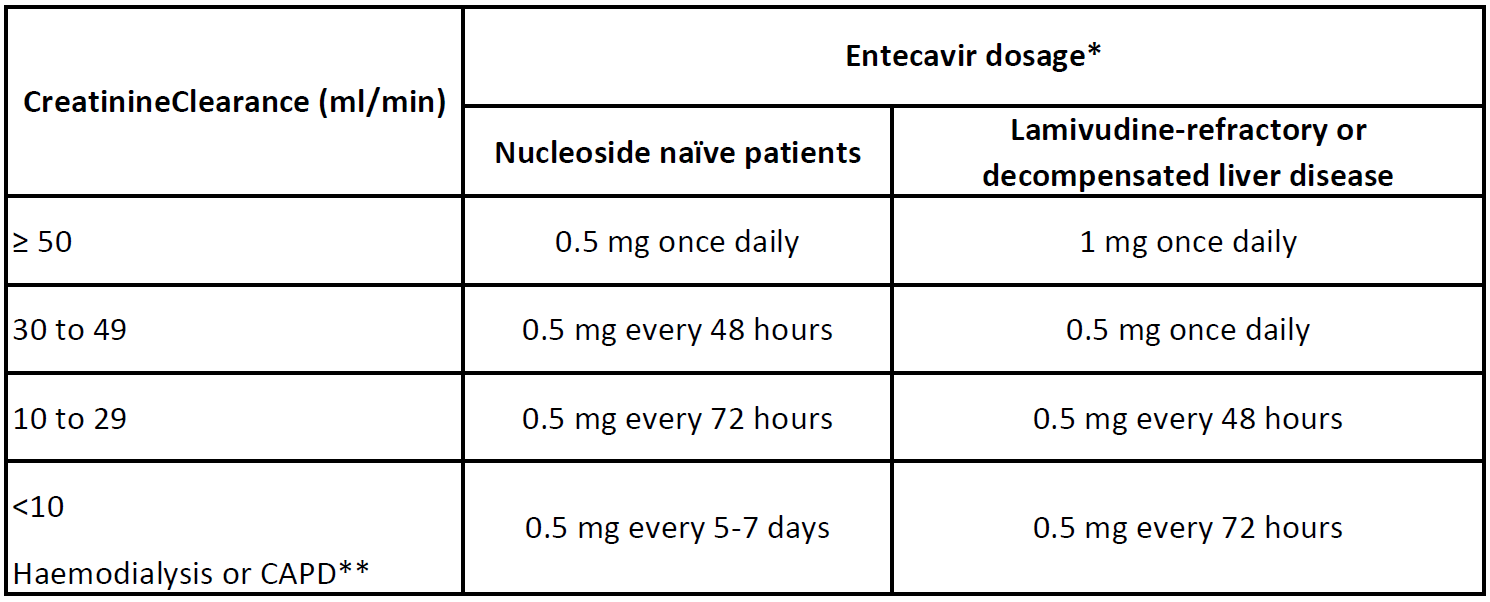
** on haemodialysis days, administer entecavir after haemodialysis.
Hepatic impairment: No dose adjustment is required in patients with hepatic Impairment.
Method of administration
Entecavir should be taken orally.
CONTRAINDICATIONS
Hypersensitivity to the active substance or to any of the excipients present in this formulation.
WARNINGS AND PRECAUTIONS FOR USE
Renal impairment: Dosage adjustment is recommended for patients with renal impairment. The proposed dose modifications are based on extrapolation of limited data, and their safety and effectiveness have not been clinically evaluated. Therefore, virological response should be closely monitored.
Exacerbations of hepatitis: Spontaneous exacerbations in chronic hepatitis B are relatively common and are characterized by transient increases in serum ALT. After initiating antiviral therapy, serum ALT may increase in some patients as serum HBV DNA levels decline. Among entecavir-treated patients on treatment exacerbations had a median time of onset of 4-5 weeks. In patients with compensated liver disease. these increases in serum ALT are generally not accompanied by an increase in serum bilirubin concentrations or hepatic decompensation. Patients with advanced liver disease or cirrhosis may be at a higher risk for hepatic decompensation following hepatitis exacerbation, and therefore should be monitored closely during therapy.
Acute exacerbation of hepatitis has also been reported in patients who have discontinued hepatitis B therapy. Post-treatment exacerbations are usually associated with rising HBV DNA, and the majority appear to be self-limited. However, severe exacerbation, including tala., have been reported.
Among entecavir-treated nucleoside naive patients, post-treatment exacerbations had a median time to onset of 23-24 weeks, and most were reported in HBeAg negative patients. Hepatic function should be monitored at repeated intervals with both clinical and laboratory follow-up for at least 8 months after discontinuation of hepatitis B therapy. If appropriate, resumption of hepatitis B therapy may be warranted.
Patients with decompensated liver disease: A higher rate of serious hepatic adverse events (regardless of causality) has been observed in patients with decompensated liver disease, in particular in those with Child Turcotte Pugh (CTP) class C disease, compared with rates in patients with compensated liver function. Also, patients with decompensated Iver disease may be at higher risk for lactic acidosis and for specific renal adverse events such as hepatorenal syndrome. Therefore, clinical and laboratory parameters should be closely monitored in this patient population.
Lactic acidosis and severe hepatomegaly with steatosis: Occurrences of lactic acidosis (in the absence of hypoxaemia), sometimes fatal. usually associated with severe hepatomegaly and hepatic steatosis, have been reported with the use of nucleoside analogues. As entecavir is a nucleoside analogue, this risk cannot be excluded. Treatment with nucleoside analogues should be discontinued when rapidly elevating aminotransferase levels, progressive hepatomegaly or metabolic/lactic acidosis of unknown aetiology occurs. Benign digestive symptoms, such as nausea, vomiting, and abdominal pain, right be indicative of lactic acidosis development. Severe cases, sometimes with fatal outcomes, were associated with pancreatitis, liver failures/hepatic steatosis, renal failure, and higher levels of serum lactate. Caution should be exercised when prescribing nucleoside analogues to any patient (particularly obese women) with hepatomegaly, hepatitis or other known risk factors for Iver disease. These patients should be followed closely.
To differentiate between elevations in aminotransferases due to response to treatment and increases potentially related to lactic acidosis, physicians should ensure that changes in ALT are associated with improvements in other laboratory markers of chronic hepatitis B.
Resistance and specific precaution for lamivudine-refractory patents: Mutations in the HEV polymerase that encode lamivudine- resistance substitutions may lead to the subsequent emergence of secondary substations, including those associated with entecavir-associated resistance (ETVr). In a small percentage of lamivudine-refractory patients, ETVr substitutions at reduces rfT184, rtS202, or rtM250 were present at baseline. Patients with lamivudine-resistant HBV are at higher risk of developing subsequent entecavir resistance than patients without lamivudine-resistant. The cumulative probability of emerging genotypic entecavir resistance after 1. 2. 3, 4, and 5 years of treatment in the lamivudine refractory studies was 6%, 15%, 36%, 47%, and 51%, respectively. The virological response should be frequently monitored in the lamivudine-refractory population and appropriate resistance testing should be performed. In patients with a suboptimal virological response after 24 weeks of treatment with entecavir, a modification of Iraiment should be considered, When starting therapy in patients with a documented history of lamivudine-resistant MBV, a combination use of entecavir plus a second antiviral agent (which does not share cross-resistance with either lamivudine or entecavir should be considered in preference to entecavir monotherapy.
Pre-existing lamivudine-resistant HBVis associated with an increased risk for subsequent entecavir resistance regardless of the degree a liver disease, in patients with decompensated liver disease, a virologic breakthrough may be associated with serious clinical complications of the underlying liver disease. Therefore, in patients with both decompensated liver disease and lamivudine-resistant HBV. combination use of entecavir plus a second antiviral agent (which does not share cross-resistance with either lamivudine or entecavir should be considered in preference to entecavir monotherapy.
Paediatric population: A lower rate of virologic response (HBV DNA < 50 lU/mL) was observed in paediatric patients with baseline HBV DNA ≥ 8.0 log 10 IU/mL. Entecavir should be used in those patients only if the potential benefit justifies the potential risk to the child (eg. resistance). Since some prediatric patients may require long-term er aven lifetime management of chronic active hepatitis B, consideration should be given to the Impact of entecavir on future treatment options.
Liver transplant recipients: Renal function should be carefully evaluated before and during entecavir therapy in liver transplant recepients receiving cyclosporine or tacrolimus.
Co-infection with hepatitis C or D: There are no data on the efficacy of entecavir in patients co-infected with hepatitis C or D virus.
Human Immunodeficiency Virus (HIV)/HBV co-infected patients not receiving concomitant antiretroviral therapy: Entecavir has not been evaluated in HIV/HBV co-infected patients not concurrently receiving effective HIV treatment. Emergence of HIV resistance has been observed when entecavir was used to treat chronic hepatitis B infection in patients with HIV infection not receiving highly active antiretroviral therapy (HAART). Therefore, therapy with entecavir should not be used for HIV/HBV co-infected patients who are not receiving HAART. Entecavir has not been studied as a treatment for HIV infection and is not recommended for this use.
HIV/HBV co-infected patients receiving concomitant antiretroviral therapy: Entecavir has been studied in 68 adults with HIV/HBV co-infection receiving a lamivudine-containing HAART regimen. No data are available on the efficacy of entecavir in HBeAg-negative patients co-infected with HIV. There are limited data on patients co-infected with HIV who have low CD4 cell counts (< 200 cells/mm3).
General: Patients should be advised that therapy with entecavir has not been proven to reduce the risk of transmission of HBV and therefore appropriate precautions should still be taken.
INTERACTIONS
Since entecavir is predominantly eliminated by the kidney, coadministration with medicinal products that reduce renal function or complete for active tubular scretion may increase, serum concentrations of either medicinal product. Apart from lamivudine, adefovir dipivoxil and tenofovir disoproxil fumarate, the effects of coadministration of entecavir with medicinal products that are excreted renally or affect renal function have not been evaluated, Patients should be monitored closely for adverse reactions when entecavir is coadministered with such medicinal products.
No pharmacokinetic interactions between entecavir and lamivudine, adefovir, or tenofovir were observed.
Entecavir is not a substrate, an inducer, or an inhibitor of cytochrome P450 (CYP450) enzymes. Therefore CYP450 mediated drug
interactions are unlikely to occur with entecavir.
PREGNANCY AND LACTATION
Pregnancy
The potential risk for humans is unknown. Entecavir should not be used during pregnancy unless clearly necessary.
Breast-feeding
It is unknown whether entecavir is excreted in human milk. Breast-feeding should be discontinued during treatment with Entecavir.
EFFECTS ON ABILITY TO DRIVE AND USE MACHINE
No studies on the effects on the ability to drive and use machines have been performed. Dizziness, fatigue, and somnolence are common side effects which may impair the ability to drive and use machines.
UNDESIRABLE EFFECTS
The most common adverse reactions of any severity with at least a possible relation to entecavir were headache (9%), and fatigue (6%). dizziness (4%) and nausea (3%). Exacerbations of hepatitis during and after discontinuation of entecavir therapy have also been reported.
Adverse reactions considered at least possibly related to treatment with entecavir are listed by body system organ class. Frequency Is defined as very common (≥ 1/10); common (≥ 1/100 to < 1/10); uncommon (2 1/1,000 to < 1/100); rare 2 1/10,000 to < 1/1,000).
Within each frequency grouping, undesirable effects are presented in order of decreasing seriousness.
Immune system disorders: rare: anaphylactoid reaction
Psychiatric disorders: common: Insomnia
Nervous system disorders: common: headache, dizziness, somnolence
Gastrointestinal disorders: common: vomiting, diarrhoea, nausea, dyspepsia
Hepatobiliary disorders: common: increased transaminases
Skin and subcutaneous tissue disorders: uncommon: rash, alopecia
General disorders and administration site conditions: common: fatigue
Exacerbations during treatment: Periodic monitoring of hepatic function is recommended during treatment.
Exacerbations after discontinuation of treatment: In the clinical trials entecavir treatment was discontinued if patients achieved a prespecified response. If treatment is discontinued without regard to treatment response, the rate of post-treatment ALT fares could be higher.
OVERDOSE
There Is limited experience of entecavir overdose reported in patients. Healthy subjects who received up to 20 mg/day for up to 14 days, and single doses up to 40 mg had no unexpected adverse reactions. If overdose occurs, the patient must be monitored for evidence of toxicity and given standard supportive treatment as necessary.
PRESENTATION:
30 Tablets packed in a HDPE container.
STORAGE:
Store below 30°C. Protect from light and moisture.
Zffam Entec 0.5: Myanmar Reg. No. : R2304AA5694
Zffam Entec 1: Myanmar Reg. No. : R2304AA5695
Product of:
Zifam Pinnacle Pty. Ltd.,
Sydney, Australia
Manufactured by:
Sai Mirra Innopharm Pvt. Ltd.
288 & 299, SIDCO Estate, Chennai – 600 098, India





