Becef
- ENG
- မြန်မာ
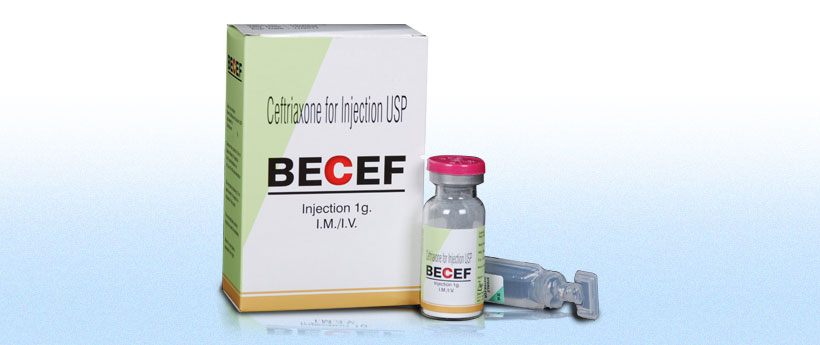
Each BECEF vial contains:
Ceftriaxone Sodium USP (Sterile)
equivalent to Anhydrous Ceftriaxone 1.0g
DECRIPTION
Ceftriaxone is bactericidal. Like other betalactams, Ceftriaxone kills bacteria by preventing their cell wall synthesis. Apart from its antimicrobial activity ceftriaxone has no clinical or pharmacological effects on any system in the body. Ceftriaxone is bactericidal at concentrations easily achievable in the plasma and tissues. It has also been suggested that betalactams may also inhibit protein and bacterial DNA synthesis.
PHARMACOKINETICS
Absorption: Ceftriaxone is not absorbed after oral administration. But it is completely absorbed following intramuscular (IM) administration with peak levels occurring 2-4 hours after the dose. Ceftriaxone is reversibly bound to plasma proteins with 95% at Plasma concentrations of less than 25 mcg/ml and 85% at 300 mcg/ml. Binding is less in neonates and children. Ceftriaxone penetrates well into most tissues and body including CSF (with inflamed and non-inflamed meninges), sputum, pleural and peritoneal fluids, ascitic fluid, tears blister fluid, bile, gall bladder Wall, bone myometrium, endomyometrium fallopian tubes, prostate, nasal mucosa, tonsil, middle ear mucosa and synovial fluid. Ceftriaxone is the first systemic cephalosporin to penetrate adequately into vitreous humour. Ceftriaxone is not metabolized in the body 33 to 67% of the dose of Ceftriaxone is excreted unchanged in the urine. The rest is secreted into the bile and ultimately excreted in the faeces as inactive compounds.
INDICATIONS
Infections of the respiratory and urinary tracts, skin and soft tissues, bones and joints. Serious bacterial infections with bacteraemia and septicaemia, Bacterial meningitis, pelvic inflammatory disease, STD and surgical prophylaxis.
CONTRAINDICATIONS
Known Hypersensitivity to cephalosporins. Hyperbilirubinaemic neonates especially premature.
DOSAGE & ADMINISTRATION
Becef may be administered intravenously or intramuscularly.
Do not use diluents containing calcium, such as Ringer’s solution or Hartmann’s solution,
Adults- 1g to 2g once a day (or equally divided doses twice a day) up to a maximum of 4g/day. Gonorrhoea a single lm dose of 250mg
Children– 50 to 75 mg/kg/day up to a maximum of 2g/day. Meningitis 100 mg/kg/day up to 4g/day.
Reconstitute Becef 1 g in 9.6 ml of appropriate diluentfor IV administration (or)1 g in 3.6mL of diluent for IM. The diluents may be sterile water for injection, 0.9% sodium chloride or 5% dextrose. Deep intragulteal muscle injection is recommended to minimize pain.
ADVERSE EFFECTS
Ceftriaxone is generally well tolerated. Adverse reactions are usually mild and transient and drug discontinuations has been required in about 1% of patients.
Common side effects have been diarrhoea, occasional nausea/vomiting, glossitis or stomatitis, hypersensitivity reactions such as rash, pruritus, urticaria and oedema transient pain at site of IM injections, local phlebitis.
WARNINGS AND PRECAUTIONS
Hypersensitivity to cephalosporin and/or penicillin should be looked before starting Ceftriaxone. If the former is known, Ceftriaxone should not be given.
OVERDOSAGE AND MANAGEMENT
One case of acute over dosage has been reported in which 100 times the recommended dose of Ceftriaxone was given intrathecally.
Dialysis does not remove Ceftriaxone from circulation to any useful extent.
DRUG INTERACTIONS
Alcohol: A possible disulfiram-like reaction may occur with alcohol, though the potential for this and hypoprothrombinaemia is low because of the N-methylthiotetrazole side chain present in cephamandole.
Diagnostic interference: Ceftriaxone does not interface with HPLC estimations of theophylline. But it has given low readings in clinic test for urine sugar. There is no interference with Diastrix or Tes tape.
PRESENTATION
Box containing 1g Becef vial with water for injection.
Updated on 9th Feb 2024