DACATEC 60 mg
- ENG
- မြန်မာ

Composition:
Each film coated tablet contains:
Daclatasvir Dihydrochloride 65.92 mg
Equivalent to Daclatasvir 60 mg
Excipients: q. s
Colour: Indigo Carmine Aluminium Lake, Yellow Iron Oxide, Titanium Dioxide BP
Pharmacological action:
Daclatasvir is an inhibitor of nonstructural protein 5A (NS5A), a multifunctional protein that is an essential component of the hepatitis C virus (HCV) replication complex. Daciatasvir inhibits both viral RNA replication and virion assembly, Identification of NS5A as the drug target was based on inhibitor-binding and mapping, inhibitor-induced resistant mutations, and crystal structure modeling. Results indicate that Daclatasvir acts at the N-terminus of the protein.
The pharmacokinetic properties of Daclatasvir were evaluated in healthy adult subjects and in patients with chronic HCV. Following multiple oral doses of Daclatasvir 60 mg once daily in combination with peginterferon alfa and ribavirin in treatment-naive patients with genotype 1 chronic HCV, the geometric mean (CV%) Daclatasvir C max was 1534 (58) ng/ml, AUC
0-24h was 14122(70) ng°h/ml, and Cmin was 232 (83) ng/ml.
Daclatasvir administered as a tablet was readily absorbed following multiple oral doses with peak plasma concentrations occurring between 1 and 2 hours.
Absorption: Daclatasvir C max, AUC, and C min increased in a near dose-proportional manner. Steady-state was achieved after 4 days of once-daily administration. At the 60 mg dose, exposure to Daclatasvir was similar between healthy subjects and HCV-infected patients.
In vitro and in vivo studies showed that Daclatasvir is a substrate of P-gp.
The absolute bioavailability of the tablet formulation is 67%.
Effect of food on oral absorption: In healthy subjects, administration of Daclatasvir 60 mg tablet after a high-fat meal decreased Daclatasvir C max and AUC by 28% and 23%, respectively, compared with administration under fasting conditions. Administration of Daclatasvir 60 mg tablet after a light meal resulted in no reduction in Daclatasvir exposure.
Therapeutic category: Antiviral
Therapeutic Indication:
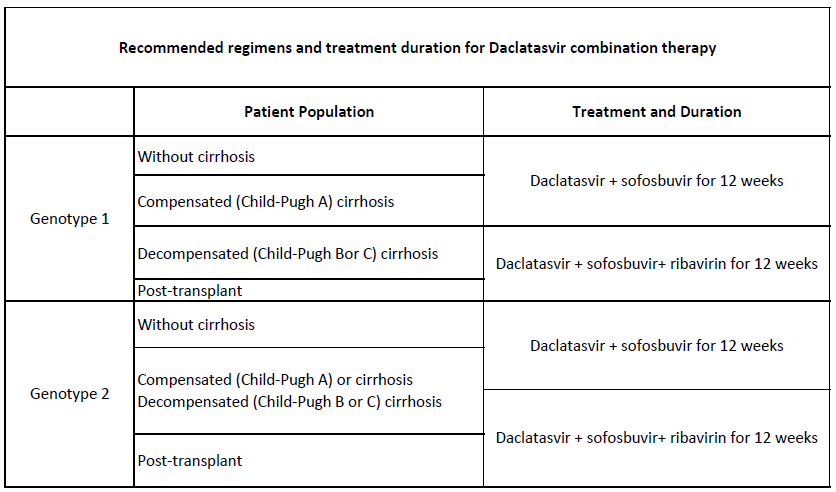
Dacatec (Daclatasvir) is indicated in combination with other medicinal products for the treatment of chronic HCV infection in adults.
Posology and Method of Administration:
Treatment with Dacatec (Daclatasvir) should be initiated and monitored by a physician experienced in the management of chronic hepatitis C.
Posology
The recommended dose of Dacatec (Daclatasvir) is 60 mg once daily, to be taken orally with or without meals.
Dacatec (Daclatasvir) must be administered in combination with other medicinal products. The Summary of Product Characteristics for the other medicinal products in the regimen should also be consulted before initiation of therapy with Dacatec (Daclatasvir).
Dose modification of Dacatec (Daclatasvir) to manage adverse reactions is not recommended. If treatment interruption of components in the regimen is necessary because of adverse reactions,
Dacatec (Daclatasvir) must not be given as monotherapy.
There are no virologic treatment stopping rules that apply to the combination of Dacatec (Daclatasvir) with sofosbuvir.
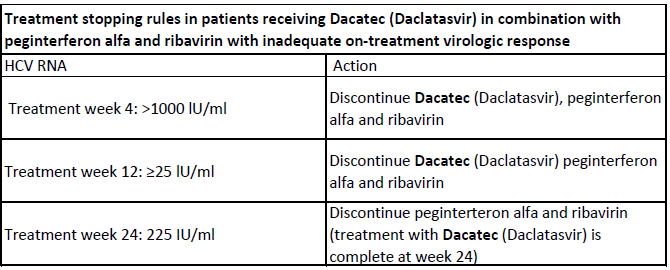
It is unlikely that patients with inadequate on-treatment virologic response will achieve a sustained virologic response (SVR); therefore discontinuation of treatment is recommended in these patients. The HCV RNA thresholds that trigger discontinuation of treatment (i.e. treatment stopping rules) are presented in Table
The dose of Dacatec (Daclatasvir) should be reduced to 30 mg once daily when coadministered with strong inhibitors of CYP3A4 and 90 mg once daily when coadministered with moderate inducers of CYP3A4
Patients should be instructed that, if they miss a dose of Dacatec (Daclatasvir), the dose should be laken as soon as possible if remembered within 20 hours of the scheduled dose time. However, if the missed dose is remembered more than 20 hours after the scheduled dose, the dose should be skipped and the next dose taken at the appropriate time.
Method of administration:
Dacatec (Daclatasvir) is to be taken orally with or without meals. Patients should be instructed to swallow the tablet whole. The film-coated table should not be chewed or crushed due the unpleasant taste of the active substance.
Contraindications:
– Hypersensitivity to the active substance or to any of the excipients.
– Coadministration with medicinal products that strongly induce cytochrome P450 3A4 (CYP3A4) and P-glycoprotein transporter (P-gp) and thus may lead to lower exposure and loss of efficacy of Daclatasvir. These active substances include but are not limited to phenytoin, carbamazepine, oxcarbazepine, phenobarbital, rifampicin, rifabutin, rifapentine, systemic dexamethasone, and the herbal product St John’s wort (Hypericum perforatum).
Warning & Precautions:
Dacatec (Daclatasvir) must not be administered as monotherapy.
Dacatec (Daclatasvir) must be administered in combination with other medicinal products for the treatment of chronic HCV infection. Should concomitant use of amiodarone be considered necessary it is recommended that patients are closely monitored when initiating Dacatec (Daclatasvir) in combination with sofosbuvir. All patients receiving Dacatec (Daclatasvir) and sofosbuvir in combination with amiodarone with or without other drugs that lower heart rate should also be warned of the symptoms of bradycardia and heart block and should be advised to seek medical advice urgently should they expenence them.
Dacatec (Daclatasvir) has not been studied in patients with HCV genotypes 5 and 6, and no regimen recommendation can be given.
Pregnancy
There are no data from the use of Daclatasvir in pregnant women. Studies of Daclatasvir in animals have shown embryotoxic and teratogenic effects.
The potential risk for humans is unknown.
Dacatec (Daclatasvir) should not be used during pregnancy or in women of childbearing potential not using contraception. Use of highly effective contraception should be continued for 5 weeks after completion of Dacatec (Daclalasvir) therapy.
Since Dacatec (Daclatasvir) is used in combination with other agents, the contraindications and warnings for those medicinal products are applicable: For detailed recommendations regarding pregnancy and contraception, refer to the Summary of Product Characteristics for ribavirin and peginterferon alfa. It is not known whether Daclatasvir is excreted in human milk. Available pharmacokinetic and toxicological data in animals have shown excretion of Daclatasvir and metabolites in milk. A risk to the newborn/infant cannot be excluded. Mothers should be instructed not to breastfeed if they are taking Dacatec (Daclalasvir).
Adverse effects: Dacatec (Daclatasvir) in combination with sofosbuvir The most frequently reported adverse reactions were fatigue, headache, and nausea. No Grade 3 or 4 adverse reactions were reported. Dacatec (Daclatasvir) in combination with peginterferon alfa and ribavirin The most frequently reported adverse reactions were fatigue, headache, pruritus, anaemia, influenza-like Illness, nausea, insomnia, neutropenia, asthenia, rash, decreased appetite, dry skin, alopecia, pyrexia, myalgia, irritability, cough, diarrhoea, dyspnoea and arthralgia.
Dizziness has been reported during treatment with Dacatec (Daclatasvir) in combination with sofosbuvir, and dizziness, disturbance in attention. blurred vision and reduced visual acuity have been reported during treatment with Dacatec (Daclalasvir) in combination with peginterferon alfa and ribavirin.
Drug Interactions:
Coadministration of Dacatec (Daclatasvir) can alter the concentration of other medicinal products and other medicinal products may alter the concentration of Daclatasvir.
The dose of Dacatec (Daclatasvir) should be reduced to 30 mg once daily when coadministered with boceprevir, telaprevir, atazanavir/ritonavir, cobicistat, atazanavir/cobicistat, clarithromycin, telithromycin, ketoconazole or other strong inhibitors of CYP3A4.
The dose of Dacatec (Daclatasvir) should be increased to 90 mg once daily when coadministered with efavirenz. Due to the lack of data, coadministration of Dacatec (Daclatasvir) and etravirine or nevirapine is not recommended. Administration of Dacatec (Daclatasvir) with erythromycin may result in increased concentrations of Daclatasvir. Caution is advised.