DOMTAF
- ENG
- မြန်မာ
For the use of a Registered Medical Practioners or Hospital only.
DOMTAF
Emtricitabine 200mg, Dolutegravir 50mg
and Tenotovir Alatenamide 25mg lablets
Composition:
Each film coated tablet contains:
Emtricitabine 200 mg
Dolutegravir Sodium
Equivalent to Dolutegravir 50 mg
Tenofovir Alafenamide Hemifumarate
Equivalent to Tenofovir Alafenamide 25 mg
Excipients: q.s.
Colour: Approved colour used.
INDICATIONS AND USAGE
DOMTAF is a three-drug combination of Dolutegravir, a human immunodeficiency virus type 1 (HIV-1) integrase strand transfer inhibitor (INSTI), and emtricitabine (FTC) and tenofovir alafenamide (TAF), both HIV-1 nucleoside analog reverse transcriptase inhibitors (NRT|s), and is indicated as a complete regimen for the treatment of HiV -1 infection in adults and pediatric patients aged 12 years and Older and weighing at least 40 kg who have no antiretroviral treatment history or to replace the current antiretroviral regimen in those who are virologically suppressed (HIV-1 RNA less than 50 copies per mL) on a stable antiretroviral regimen with no history of treatment faiture and no known substitutions associated with resistance to the individual components of DOMTAF
DOSAGE AND ADMINISTRATION
• Testing: Prior to or when initiating DOMTAF Test for Hepatitis B Virus infection. Prior to or When Initiating DOMTAF, and During Treatment, assess Serum Creatinine, estimated Creatinine Clearance, Urine Glucose, and urine protein in all patients as clinically appropriate. In patients with Chronic kidney disease, also assess Serum Phosphorus.
• Recommended Dose: One Tablet once daily with or without food
• Renal Impairment: DOMTAF is not recommended in patients with estimated renal creatinine clearance below 30 mL per minute
• Hepatic Impairment: DOMTAF is not recommended in patients with severe hepatic impairment.
CONTRAINDICATIONS
DOMTAF is contraindicated to be co-administered with:
Dofetilide due to the potential for increased Dofetilide plasma concentrations and associated serious and/or life-threatening events.
WARNINGS AND PRECAUTIONS
• Immune Reconstitution Syndrome has been reported in patients treated with combination antiretroviral therapy. During the initial phase of combination antiretroviral treatment, patients whose immune system responds may develop an inflammatory response to indolent or residual opportunistic infections, which may necessitate further evaluation and treatment.
• Hypersensitivity reactions characterised by rash, constitutional findings, and sometimes organ dysfunction, including liver injury, have been reported. Discontinue DOMTAF if signs or symptoms of hypersensitivity reactions develop.
• Patients with underlying hepatitis B or C may be at increased risk for worsening or development of transaminase elevations with use of
DOMTAF. Appropriate laboratory testing prior to initiating therapy and monitoring for hepatotoxicity during therapy with DOMTAF is recommended in patients with underlying hepatic disease such as hepatitis B or C.
• New onset or worsening renal impairment: Assess creatinine clearance, urine protein in all patients before initiating DOMTAF therapy and monitor during therapy. Monitor serum Phosphorus in patients with Chronic kidney disease.
• Lactic acidosis/ severe hepatomegaly with stealosis: Discontinue treatment in patients who develop symptoms or laboratory findings suggestive of lactic acidosis or Pronounced hepatoxicity.
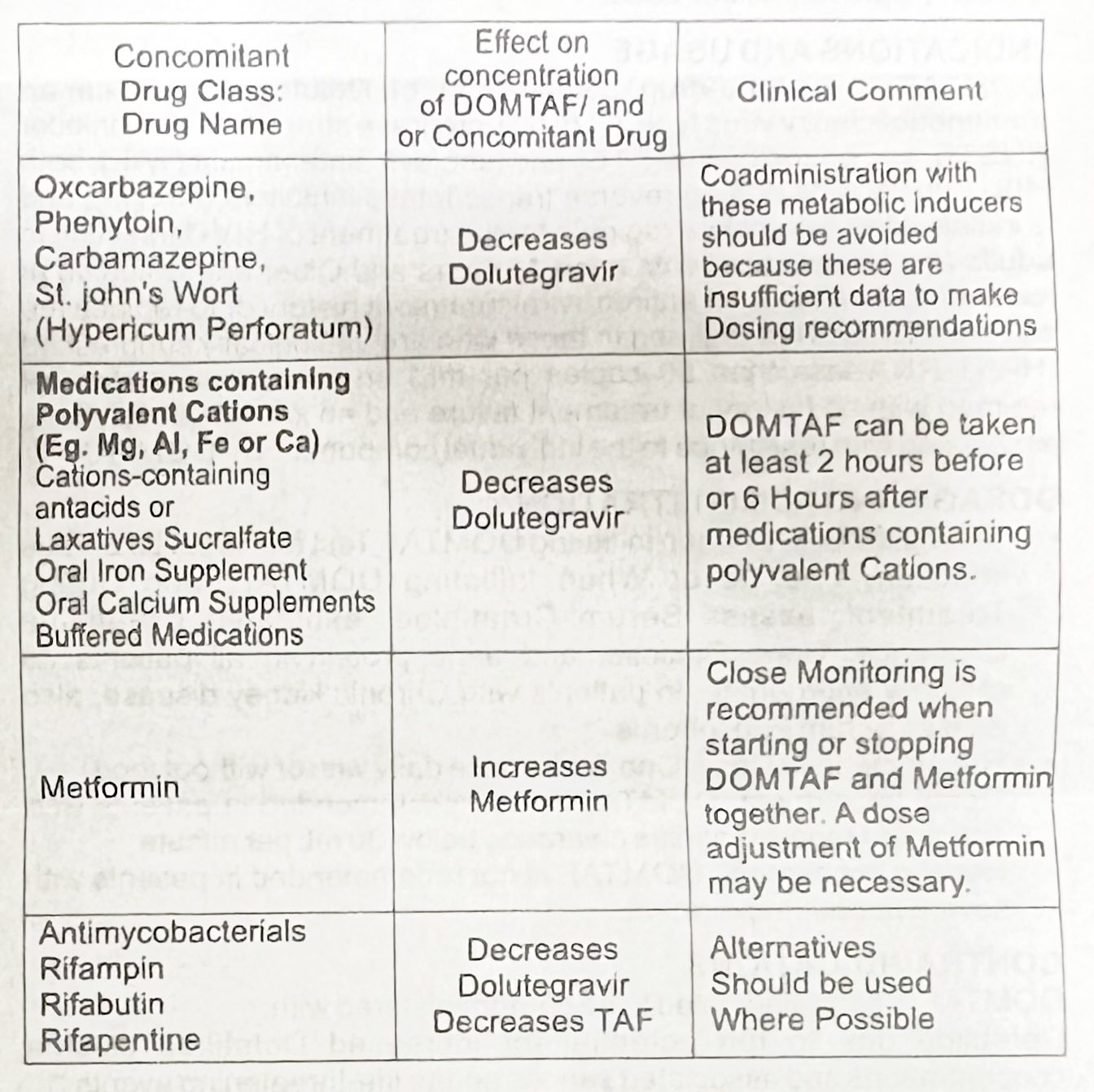
DRUG INTERACTION
• DOMTAF Is a complete regimen, coadministration with other antiretroviral medications for the treatment of HIV-1 infection in not recommended
• DOMTA inhibits renal organic cation transporter 2 (OCT2), coadministration of DOMTAF with Drugs that are substrates of OCT2 (ex. Dofetilide) may increase their plasma concentration.
• Dolutegravir is a substrate of CYP3A and UGT1A1. A Drug that is a strong inducer of CYP3A and also an inducer of UGT1A1 can substantially decrease the plasma concentration of Dolutegravir.
• TAF is a substrate of P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP). Co-administration of drugs that inhibit P-gp and BCRP may Increase the absorption and plasma concentration of TAF. Co-administration of drugs that induce P-gp activity are expected to decrease the absorption of TAF, resulting in decreased plasma concentration of TAF, which may lead to loss of therapeutic effect of DOMTAF and development of resistance.
Established and Potentially Significant Drug Interactions:
Alternation in Regimen may be recommended
ADVERSE REACTIONS
Most Common adverse reactions (Incidence greater than or equal to 5%, all Grades) are diarrhea, Nausea, and Headache.
USE IN SPECIAL POPULATION
• Pregnancy: DOMTAF Should Be used during pregnancy only if the potential benefit justifies the potential risk
• Nursing mothers: Breastfeeding is not recommended due to the potential for HIV Transmission
• Pediatric patients: Safety and efficacy of DOMTAF have not been established in pediatric patients younger than 12 years or weighing less than 40kg, or in pediatric patients who are INSTI – experienced with documented or clinically suspected resistance to other INSTIs
Pharmaceutical information:
Storage conditions: Store in cool and dry place below 30°C.
Protect from light and moisture. Keep medicine away from children.
Shelf Life: 24 months
Pack size: Bottle Pack of 30 Tablets
Product of:
Zifam Pinnacle Pty. Ltd.
Sydney, Australla.
Manufactured by:
FREDUN PHARMACEUTICALS LTD.
14,15,16, Zorabian Indl. Complex, Vevoor, Palghar – 401404, Maharashtra State, INDIA.