DTV 50
- ENG
- မြန်မာ
DTV 50
Dolutegravir Tablet 50mg
Composition:
Each film coated tablet contains:
Dolutegravir Sodium 52.6 mg
Equivalent to Dolutegravir 50 mg
Excipients: q.s.
Colour: Iron Oxide Yellow, Titanium Dioxide BP
Pharmacological action:
Dolutegravir inhibits HIV integrase by binding to the integrase active site and blocking the strand transfer step of retroviral Deoxyribonucleic acid (DNA) integration which is essential for the HIV replication cycle.
Pharmacodynamic effects
Antiviral activity in cell culture
The IC50 for dolutegravir in various labstrains using PBMC was 0.5 nM, and when using MT-4 cells it ranged from 0.7-2 nM. Similar IC50s were seen for clinical isolates without any major difference between subtypes; in a panel of 24 HIV-1 isolates of clades A, B, C, D, E, F and G and group O the mean IC50 value was 0.2 nM (range 0,02-2.14). The mean IC50 for 3 HIV-2 isolates was 0.18 nM (range 0.09-0.61 ).
Antiviral activity in combination with other antiviral agents
No antagonistic effects in vitro were seen with dolutegravir and other antiretrovirals tested agents: stavudine, abacavir, efavirenz, nevirapine, lopinavir, amprenavir, enfuvirtide, maraviroc and raltegravir. In addition, no antagonistic effects were seen for dolutegravir and adefovir, and ribavirin had no apparent effect on dolutegravir activity.
Effect of human serum
In 100% human serum, the mean protein fold shift was 75 fold, resulting in protein adjusted IC90 of 0.064 ug/ml
Pharmacokinetic properties
Dolutegravir pharmacokinetics are similar between healthy and HIV-infected subjects. The PK variability of dolutegravir is low to moderate. In Phase I studies in healthy subjects, between-subject CVb% for AUC and Cmax ranged from -20 to 40% and C-r from 30 to 65% across studies. The between-subject PK variability of dolutegravir was higher in HIV-infected subjects than healthy subjects. Within-subject variability (Cvw%) is lower than between-subject variability.
Pharmacokinetic/pharmacodynamic relationship
In a randomized, dose-ranging trial, HIV-1-infected subjects treated with dolutegravir monotherapy
(ING111521) demonstrated rapid and dose-dependent antiviral activity, with mean decline in HIV-1 RNA of 2.5 log10 at day 11 for 50 mg dose. This antiviral response was maintained for 3 to 4 days after the last dose in the 50 mg group
Therapeutic category: Antiviral
Therapeutic Indication:
DTV 50 is indicated in combination with other anti-retroviral medicinal products for the treatment of Human Immunodeficiency Virus (HIV) infected adults and adolescents above 12 years of age.
Posology and Method of administration:
DTV 50 should be prescribed by physicians experienced in the management of HIV infection.
Posology
Adults
Patients infected with HIV-1 without resistance to the integrase class
The recommended dose ofDTV 50 is 50 mg ( one tablet) orally once daily.
DTV 50 should be administered twice daily in this population when coad ministered with some medicines (e.g. efavirenz, nevirapine, tipranavir/ritonavir, or rifampicin).
Patients infected with HIV-1 with resistance to the integrase class
The recommended dose of DTV 50 is 50 mg (one tablet) twice daily. The decision to use dolutegravir for such patients should be informed by the integrase resistance pattern.
Co-administration of DTV 50 with some medicines should be avoided in this population (e.g. Efavirenz, nevirapine, tipranavir/ritonavir, or rifampicin).
Adolescents aged 12 and above
In adolescents (aged from 12 to 17 years and weighing at least 40 kg) infected with HIV-1 without resistance to the integrase class, the recommended dose of DTV 50 is 50 mg once daily.
Elderly
There is no evidence that elderly patients require a different dose than younger adult patients.
Hepatic and renal impairment
No dosage adjustment is required in patients with mild, moderate or severe (CrCI <30 mUmin, not on dialysis) renal impairment.
No dosage adjustment is required in patients with mild or moderate hepatic impairment. DTV 50 should be used with caution in patients with severe hepatic impairment.
Method of administration
Oral use.
DTV 50 can be taken with or without food. In the presence of integrase class resistance, DTV 50 should preferably be taken with food to enhance exposure (particularly in patients with 0148 mutations).
If the patient misses a dose of DTV 50, the patient should take DTV 50 as soon as possible, providing the next dose is not due within 4 hours. If the next dose is due within 4 hours, the patient should not take the missed dose and simply resume the usual dosing schedule.
Contraindications
DTV 50 is contraindicated in patients:
with previous hypersensitivity reaction to dolutegravir.
receiving dofetilide due to the potential for increased dofetilide plasma concentrations and the risk for serious and/or life-threatening events.
Special Warning and Precautions for Use
While effective viral suppression with antiretroviral therapy has been proven to substantially reduce the risk of sexual transmission, a residual risk cannot be excluded. Precautions to prevent transmission should be taken in accordance with national guidelines.
The decision to use DTV 50 in the presence of integrase class resistance should take into account that the activity of DTV 50 is considerably compromised for viral strains harbouring 0148+>2 secondary mutations from G140A/C/S, E138A/K/T, L74I.
Hypersensitivity reactions have been reported with DTV 50, and were characterized by rash, constitutional findings, and sometimes, organ dysfunction, including severe liver reactions. DTV 50 and other suspect agents should be discontinued immediately if signs or symptoms of hypersensitivity reactions develop.
Clinical status including liver aminotransferases and bilirubin should be monitored. Delay in stopping treatment with DTV 50 or other suspect active substances after the onset of hypersensitivity may result in a life-threatening allergic reaction.
In HIV-infected patients with severe immune deficiency at the time of institution of combination antiretroviral therapy (CART), an inflammatory reaction to asymptomatic or residual opportunistic pathogens may arise and cause serious clinical conditions, or aggravation of symptoms. Any inflammatory symptoms should be evaluated and treatment instituted when necessary.
Monitoring of liver biochemistries is recommended in some hepatitis 8 and/or C co-infected patients at the start of DTV 50 therapy. Particular diligence should be applied in initiating or maintaining effective hepatitis 8 therapy when starting DTV SO-based therapy in hepatitis 8 co-infected patients. Patients should remain under close clinical observation by physicians experienced in the treatment of associated HIV diseases like opportunistic infections.
Patients with advanced HIV-disease and/or long-term exposure to CART should be advised to seek medical advice if they experience joint aches and pain, joint stiffness or difficulty in movement.
Pregnancy
There are no or limited amount of data from the use of dolutegravir in pregnant women. The effect of dolutegravir on human pregnancy is unknown. In reproductive toxicity studies in animals, dolutegravir was shown to cross the placenta. Anim,al studies do not indicate direct or indirect harmful effects with respect to reproductive toxicity. DTV 50 should be used during pregnancy only if the expected benô€ fit justifies the potential risk to the foetus.
It is unknown whether dolutegravir is excreted in tµJman milk. Available toxicological data in animals has shown excretion of dolutegravir in milk. In lactating rats that received a single oral dose of 50 mg/kg at 10 days postpartum, dolutegravir was detected in milk at concentrations typically higher than blood. It is recommended that HIV infected women do not breast-feed their infants under any circumstances in order to avoid transmission of HIV.
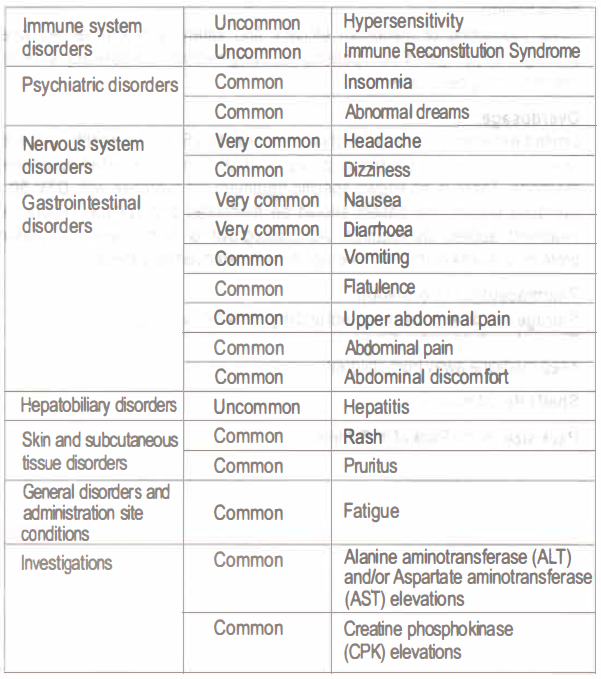
Adverse effects:
The most severe adverse reaction was a hypersensitivity reaction that included rash and severe liver effects. The most commonly seen treatment emergent adverse reactions were nausea, diarrhoea and headache. The adverse reactions possibly considered due to dolutegravir on body system, organ class with absolute frequency are listed below:
Drug Interactions:
All factors that decrease dolutegravir exposure should be avoided in the presence of integrase class resistance.
Dolutegravir is eliminated mainly through metabolism by UGT1A 1. Dolutegravir is also a substrate of UGT1A3, UGT1A9, CYP3A4, Pgp, and BCRP; therefore medicinal products that induce those enzymes may decrease dolutegravir plasma concentration and reduce the therapeutic effect of dolutegravir. CoÂadministration of dolutegravir and other medicinal products that inhibit these enzymes may increase dolutegravir plasma concentration.
The absorption of dolutegravir is reduced by certain anti-acid agents.
In vitro, dolutegravir demonstrated no direct, or weak inhibition (IC50>50 µM) of the enzY411es cytochrome P450 (CYP)1A2, CYP2A6, CYP286, CYP2C8, CYP2C9, CYP2C19, CYP2D6 CYP3A, uridine diphosphate glucuronosyl transferase (UGT)1A1 or UGT287, or the transporters Pgp, BCRP, BSEP, OATP1B1, OATP183, OCT1, MATE2-K, MRP2 or MRP4. In vitro, dolutegravir did not induce CYP1A2, CYP286 or CYP3A4. In vivo, dolutegravir does not seem to have an effect on midazolam, a CYP3A4 probe, however, a weak inhibition can presently not be excluded.
Etravirine decreased plasma dolutegravir concentration, which may result in loss of virologic response and possible resistance to dolutegravir. DTV 50 should not be used with etravirine without co-administration of atazanavir/ritonavir, darunavir/ritonavir or lopinavir/ritonavir.
The recommended dose of DTV 50 is 50 mg twice daily when co-administered with efavirenz, nevirapine, rifampicin, tipranavir/ritonavir and fosamprenavir/ritonavir with the absence of integrase class resistance. In the presence of integrase class resistance these combinations should be avoided. DTV 50 and dofetilide co-administration is contraindicated due to potential lifethreatening toxicity caused by high dofetilide concentration. Co-administration with these enzyme inducers should be avoided. No dose adjustment is necessary. Based on data from other CYP3A4 inhibitors, a marked increase is not expected.
Co-administration with St. John’s wort is strongly discouraged.
Magnesium/ aluminium-containing antacid should be taken well separated in time from the administration of DTV 50 (minimum 2 hours after or 6 hours before).
Calcium supplements, iron supplements or multivitamins should be taken well separated in time from the administration of DTV 50 (minimum 2 hours after or 6 hours before).
Close monitoring of metformin efficacy and safety is recommended when starting or stopping DTV 50 in patients receiving metformin. A dose adjustment of metformin may be necessary.
Overdosage:
Limited experience of single higher doses (up to 250 mg in healthy subjects) revealed no specific symptoms or signs, apart from those listed as adverse reactions. There is no known specific treatment for overdose with DTV 50. If overdose occurs, the patient should be monitored and standard supportive treatment applied as required. As dolutegravir is highly bound to plasma proteins, it is unlikely that it will be significantly removed by dialysis.
Pharmaceutical information:
Storage conditions: Store in cool and dry place below 30°C.
Protect from light and moisture.
Keep medicine away from children.
Shelf Life: 24 months
Pack size: Bottle Pack of 30 Tablets
Product of:
Zifam Pinnacle Pty Ltd.,
Sydney, Australia.
Manufactured by:
Fredun Pharmaceuticals Ltd.
14, 15, 16, Zorabian l ndl. Complex, Vevoor,
Palghar- 401404, Maharashtra State, INDIA.