Faxofer 40
- ENG
- မြန်မာ
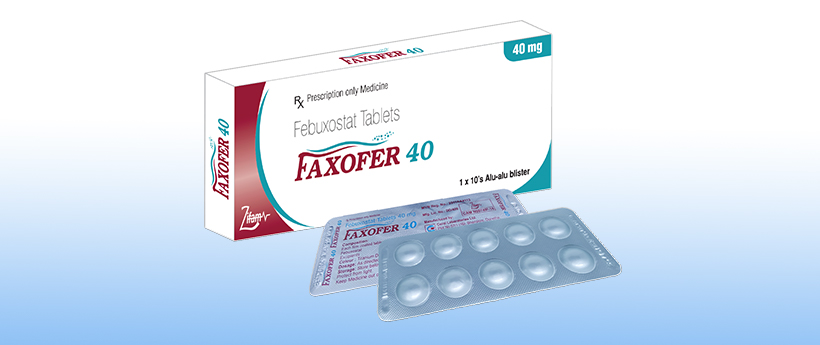
Composition:
Each film coated tablet contains:
Febuxostat ……… 40 mg
Excipients……… q.s.
Colour : Titanium Dioxide
PHARMACOLOGY:
Febuxostat belongs to the pharmacotherapeutic group: Antigout preparation, preparations inhibiting uric acid production (ATC code: M04A03).
Mechanism of action
Uric acid is the end product of purine metabolism in humans and is generated in the cascade of hypoxanthine & xanthine & uric acid. Both steps in the above transformations are catalyzed by xanthine oxidase (X). Febuostat is a 2-arylthiazole derivative that achieves its therapeutic effect of decreasing serum uric acid by selectively inhibiting XO.
Febuxostat is a potent, non-purine selective inhibitor of XO (NP-SIXO) with an in vitro inhibition Ki value less than one nanomolar. Febuxostat has been shown to potently inhibit both the oxidized and reduced forms of XO. At therapeutic concentrations febuxostat does not inhibit other enzymes involved in purine or pyrimidine metabolism, namely, guanine deaminase, hypoxanthine guanine phosphoribosyltransferase, orotate phosphoribosyltransferase, orotidine monophosphate decarboxylase or purine nucleoside phosphorylase.
Pharmacokinetics
In healthy subjects, maximum plasma concentrations (Cmax) and area under the plasma concentration time curve (AUC) of febuxostat increased in a dose proportional manner following single and multiple doses of 10 mg to 120 mg. For doses between 120 mg and 300 mg, a greater than dose proportional increase in AUC is observed for febuxostat. There is no appreciable accumulation when doses of 10 mg to 240 mg are administered every 24 hours. Febuxostat has an apparent mean terminal elimination half-life (t1/2) of approximately 5 to 8 hours. Population pharmacokinetic/pharmacodynamic analyses were conducted in 211 patients with hyper uricaemia and gout, treated with febuxostat 40-240 mg QD. In general, febuxostat pharmacokinetic parameters estimated by these analyses are consistent with those obtained from healthy subjects, indicating that healthy subjects are representative for pharmacokinetic / pharmacodynamic assessment in the patient population with gout.
Absorption: Febuxostat is rapidly (tax of 1.0-1.5 h) and well absorbed (at least 84%). After single or multiple oral 80 and 120 mg once daily doses, Cmax is approximately 2.8-3.2
4g/mL, and 5.0-5.3 ug/mL, respectively. Absolute bioavailability of the febuxostat tablet formulation has not been studied.
Following multiple oral 80 mg once daily doses or a single 120 mg dose with a high fat meal, there was a 49% and 38% decrease in Cmax and a 18% and 16% decrease in AUC, respectively. However, no clinically significant change in the percent decrease in serum uric acid concentration was observed where tested (80 mg multiple dose). Thus, febuxostat may be taken without regard to food.
Distribution: The apparent steady state volume of distribution (Vss/F) of tebuxostat ranges from 29 to 75 L after oral doses of 10-300 mg. The plasma protein binding of febuxostat is approximately 99.2%, (primarily to albumin), and is constant over the concentration range achieved with 80 and 120 mg doses. Plasma protein binding of the active metabolites ranges from about 82% to 91%.
Biotransformation: Febuxostat is extensively metabolized by conjugation via uridine diphosphate glucuronosyltransferase (UDGT) enzyme system and oxidation via the cytochrome P450 (CYP) system. Four pharmacologically active hydroxyl metabolites have been identified, of which three occur in plasma of humans. In vitro studies with human liver microsomes showed that those oxidative metabolites were formed primarily by CYP1A1, CYP1A2, CYP2C8 or CYP2C9 and febuxostat glucuronide was formed mainly by UGT 1A1, 1A8, and 1A9.
Elimination: Febuxostat is eliminated by both hepatic and renal pathways. Following an 80: mg oral dose of 14C-labeled febuxostat, approximately 49% of the dose was recovered in the urine as unchanged febuxostat (3%), the acyl glucuronide of the active substance (30%), its known oxidative metabolites and their conjugates (13%), and other unknown metabolites (3%). In addition to the urinary excretion, approximately 45% of the dose was recovered in the faeces as the unchanged febuxostat (12%), the acyl glucuronide of the active substance (1%), its known oxidative metabolites and their conjugates (25%), and other unknown metabolites (7%).
INDICATIONS:
FAXOFER 40 is a xanthine oxidase (X) inhibitor indicated for the chronic management of hyperuricemia in patients with gout.
FAXOFER 40 is not recommended for the treatment of asymptomatic hyperuricemia.
DOSAGE AND ADMINISTRATION:
For treatment of hyperuricemia in patients with gout, FAXOFER 40 is recommended at 40 mg or 80 mg once daily.
The recommended starting dose of FAXOFER 40 is 40 mg once daily. For patients who do not achieve a serum uric acid (sUA) less than 6 mg per dL after 2 weeks with 40 mg, Febuxostat 80 mg is recommended.
FAXOFER 40 can be taken without regard to food or antacid use.
Special Populations: No dose adjustment is necessary when administering Febuxostat in patients with mild to moderate renal impairment.
The recommended starting dose of Febuxostat is 40 mg once daily. For patients who do not achieve a sUA less than 6 mg per dL after 2 weeks with 40 mg, Fébuxostat 80 mg is recommended. No dose adjustment is necessary in patients with mild to moderate hepatic impairment
Uric Acid Level: Testing for the target serum uric acid level of less than 6 mg per dL may be performed as early as 2 weeks after initiating Febuxostat therapy.
Gout Flares: Gout flares may occur after initiation of Febuxostat due to changing serum uric acid levels resulting in mobilization of urate from tissue deposits. Flare prophylaxis with a non-steroidal anti-inflammatory drug (NSAID or colchicine is recommended upon initiation of Febuxostat. Prophylactic therapy may be beneficial for up to six months If a gout flare occurs during Febuxostat treatment, Febuxostat need not be discontinued.
The gout flare should be managed concurrently, as appropriate for the individual patient
CONTRAINDICATIONS:
Febuxostat is contraindicated in patients being treated with azathioprine, mercaptopurine, or theophylline.
Hypersensitivity to the active substance or to any of the excipients
WARNINGS AND PRECAUTIONS:
Gout Flare:
After initiation of Febuxostat, an increase in gout flares is frequently observed. This increase is due to reduction in serum uric acid levels resulting in mobilization of urate from tissue deposits.
In order to prevent gout flares when Febuxostat is initiated, concurrent prophylactic treatment with an NSAID or colchicine is recommended
Cardiovascular Events:
In the randomized controlled studies, there was a higher rate of cardiovascular thromboembolic events (cardiovascular deaths, non-fatal myocardial infarctions, and non-fatal strokes) in patients treated with Febuxostat [0.74 per 100 P-Y (95% CI 0.36-1.37)] than allopurinol [0.60 per 100 P-Y (95% Cl 0.16-1.53)]. A causal relationship with.
Febuxostat has not been established. Monitor for signs and symptoms of myocardial infarction (MI) and stroke.
Liver Enzyme Elevations:
During randomized controlled studies, transaminase elevations greater than 3 times the upper limit of normal (ULN) were observed (AST: 2%, 2%, and ALT: 3%, 2% in Febuxostat and allopurinol-treated patients, respectively). No dose-effect relationship for these transaminase elevations was noted. Laboratory assessment of liver function is recommended at, for example, 2 and 4 months following initiation of Febuxostat and periodically thereafter.
ADVERSE REACTIONS
Clinical Trials Experience:
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
A total of 2757 subjects with hyperuricemia and gout were treated with Febuxostat 40 mg or 80 mg daily in clinical studies. For Febuxostat 40 mg, 559 patients were treated for ≥ 6 months. For Febuxostat 80 mg, 1377 subjects were treated for ≥ 6 months, 674 patients were treated for ≥ 1 year and 515 patients were treated for ≥ 2 years, Most Common Adverse Reactions:
In three randomized, controlled clinical studies (Studies 1, 2 and 3), which were 6 to 12 months in duration, the following adverse reactions were reported by the treating physician as related to study drug. Table 1 summarizes adverse reactions reported at a rate of at least 1% in Febuxostat treatment groups and at least 0,5% greater than placebo.
| Table 1: Adverse Reactions Occurring in ≥ 1% of Febuxostat-Treated Patients and at
Least 0.5% Greater than Seen in Patients Receiving Placebo in Controlled Studios |
||||
| Adverse Reactions | Placebo | FEBUXOSTAT | Allopurinol* | |
| (N=134) | 40 mg daily
(N=757) |
80 mg daily
(N=1279) |
(N=1277) | |
| Liver Function Abnormalities | 0.7% | 6.6% | 4.6% | 4.2% |
| Nausea | 0,7% | 1.1% | 1.3% | 0.8% |
| Arthralgia | 0% | 1.1% | 0.7% | 0.7% |
| Rash | 0.7% | 0.5% | 1.6% | 1.6% |
*Of the subjects who received allopurinol, 10 received 100 mg, 145 received 200 mg, and 1122 received 300 mg, based on level of renal impairment.
The most common adverse reaction leading to discontinuation from therapy was liver function abnormalities in 1.8% of Febuxostat 40 mg, 1.2% of Febuxostat 80 mg, and in 0.9% of allopurinol-treated subjects.
In addition to the adverse reactions presented in Table 1, dizziness was reported in more than 1% of Febuxostat-treated subjects although not at a rate more than 0.5% greater than placebo.
Less Common Adverse Reactions: In phase 2 and 3 clinical studies the following adverse reactions occurred in less than 1% of subjects and in more than one subject treated with doses ranging from 40 mg to 240 mg of Febuxostat. This list also includes adverse reactions (less than 1% of subjects) associated with organ systems from Warnings and Precautions.
Blood and Lymphatic System Disorders: anemia, idiopathic thrombocytopenic purpura, leukocytosis/leukopenia, neutropenia, pancytopenia, splenomegaly, thrombocytopenia.
Cardiac Disorders: angina pectoris, atrial fibrillation/flutter, cardiac murmur, ECG abnormal, palpitations, sinus bradycardia, tachycardia.
Ear and Labyrinth Disorders: deafness, tinnitus, vertigo.
Eye Disorders: vision blurred.
Gastrointestinal Disorders: abdominal distention, abdominal pain, constipation, dry mouth, dyspepsia, flatulence, frequent stools, gastritis, gastroesophageal reflux disease, gastrointestinal discomfort, gingival pain, haematemesis, hyperchlorhydria, hematochezia, mouth ulceration, pancreatitis, peptic ulcer, vomiting.
General Disorders and Administration Site Conditions: asthenia, chest pain/discomfort, edema, fatigue, feeling abnormal, gait disturbance, influenza-like symptoms, mass, pain, thirst.
Hepatobiliary Disorders: cholelithiasis/cholecystitis, hepatic steatosis, hepatitis, hepatomegaly.
Immune System Disorder: hypersensitivity.
Infections and Infestations: herpes zoster.
Procedural Complications: contusion.
Metabolism and Nutrition Disorders: anorexia, appetite decreased/increased, dehydration, diabetes mellitus, hypercholesterolemia, hyperglycemia, hyperlipidemia, hyper triglyceri-demia, hypokalemia, weight decreased/increased.
Musculoskeletal and Connective Tissue Disorders: arthritis, joint stiffness, joint swelling, muscle spasms/twitching/tightness/weakness, musculoskeletal pain/stiffness, myalgia.
Nervous System Disorders: altered taste, balance disorder, cerebrovascular accident, Guillain-Barré syndrome, headache, hemiparesis, hypoesthesia, hyposmia, lacunar infarction, lethargy, mental impairment, migraine, paresthesia, somnolence, transient ischemic attack, tremor.
Psychiatric Disorders: agitation, anxiety, depression, insomnia, irritability, libido decreased, nervousness, panic attack, personality change.
Renal and Urinary Disorders: hematuria, nephrolithiasis, pollakiuria, proteinuria, renal failure, renal insufficiency, urgency, incontinence.
Reproductive System and Breast Changes: breast pain, erectile dysfunction, gynecomastia.
Respiratory, Thoracic and Mediastinal Disorders: bronchitis, cough, dyspnea, epistaxis, nasal dryness, paranasal sinus hypersecretion, pharyngeal edema, respiratory tract congestion, sneezing, throat irritation, upper respiratory tract infection.
Skin and Subcutaneous Tissue Disorders; alopecia, angio-edema, dermatitis, dermographism, ecchymosis, eczema, hair color changes, hair growth abnormal, hyperhidrosis, peeling skin, petechiae, photosensitivity, pruritus, purpura, skin discoloration/altered pigmentation, skin lesion, skin odor abnormal, urticaria.
Vascular Disorders: flushing, hot flush, hypertension, hypotension.
Laboratory Parameters: activated partial thromboplastin time prolonged, creatine increased, bicarbonate decreased, sodium increased, EEG abnormal, glucose increased, cholesterol increased, triglycerides Increased, amylase increased, potassium increased, TSH increased, platelet count decreased, hematocrit decreased, hemoglobin decreased, MCV increased, RBC decreased, creatinine increased, blood urea increased, BUN/creatinine ratio increased, creatine phosphokinase (CK) increased, alkaline phosphatase increased, LDH increased, PSA increased, urine output increased/decreased, lymphocyte count decreased, neutrophil count decreased, WBC increased/decreased, coagulation test abnormal, low density lipoprotein (LDL) increased, prothrombin time prolonged, urinary casts, urine positive for white blood cells and protein.
Cardiovascular Safety: Cardiovascular events and deaths were adjudicated to one of the pre-defined endpoints from the Anti-Platelet Trialists’ Collaborations (APTC) (cardiovascular death, non-fatal myocardial infarction, and non-fatal stroke) in the randomized controlled and long-term extension studies. In the Phase 3 randomized controlled studies, the incidences of adjudicated APTC events per 100 patient-years of exposure were:
Placebo 0 (95% Cl 0.00-6.16), Febuxostat 40 mg 0 (95% Cl 0.00-108), Febuxostat 80 mg 1.09 (95% Cl 0.44-2.24), and allopurinol 0.60 (95% C 0,16-1.53).
In the long-term extension studies, the incidences of adjudicated APTC events were:
Febuxostat 80 mg 0.97 (95% Cl 0.57-1.56), and allopurinol 0.58 (95% CI 0.02-3.24), Overall, a higher rate of APTC events was observed in Febuxostat than in allopurino-treat-ed patients. A causal relationship with Febuxostat has not been established. Monitor for signs and symptoms of MI and stroke.
DRUG INTERACTIONS:
Xanthine Oxidase Substrate Drugs, Febuxostat is an XO inhibitor. Drug interaction studies of Febuxostat with drugs that are metabolized by XO (e.g., theophylline, mercaptopurine, azathioprine) have not been conducted. Inhibition of XO by Febuxostat may cause increased plasma concentrations of these drugs leading to toxicity. Febuxostat is contraindicated in patients being treated with azathioprine, mercaptopurine, or theophylline.
Cytotoxic Chemotherapy Drugs: Drug interaction studies of Febuxostat with cytotoxic chemotherapy have not been conducted. No data are available regarding the safety of Febuxostat during cytotoxic chemotherapy.
In Vivo Drug Interaction Studies: Based on drug interaction studies in healthy subjects, Febuxostat does not have clinically significant interactions with colchicine, naproxen, indomethacin, hydrochlorothiazide, warfarin or desipramine. Therefore, Febuxostat may be used concomitantly with these medications.
PREGNANCY & LACTATION:
Pregnancy: Data on a very limited number of exposed pregnancies have not indicated any adverse effects of febuxostat on pregnancy or on the health of the foetus/new born child.
Animal studies do not indicate direct or indirect harmful effects with respect to pregnancy, embryonal/foetal development or parturition. The potential risk for human is unknown.
Febuxostat should not be used during pregnancy.
Breastfeeding: It is unknown whether febuxostat is excreted in human breast milk. Animal studies have shown excretion of this active substance in breast milk and an impaired development of suckling pups. A risk to a suckling infant cannot be excluded. Febuxostat should not be used while breastfeeding.
Fertility: In animals, reproduction studies up 10 48 mg/kg/day showed no dose-dependent adverse effects on fertility. The effect of Febuxostat on human fertility is unknown.
EFFECTS ON ABILITY TO DRIVE AND USE MACHINES:
Somnolence, dizziness, paraesthesia and blurred vision have been reported with the use of Febuxostat. Patients should exercise caution before driving, using machinery or participating in dangerous activities until they are reasonably certain that Febuxostat does not adversely affect performance,
OVERDOSAGE:
Patients with an overdose should be managed by symptomatic and supportive care.
STORAGE:
Store below 30°C in a dry place. Protect from light.
Keep Medicine out of reach of children.
PRESENTATION:
1 x 10 tablets in Alu – Alu blister
Updated on 9/Feb/2024