Lamitec 100
- ENG
- မြန်မာ
Composition
Lamitec 100 mg
Each film-coated tablet contains: Lamivudine USP 100mg
Pharmacological Action
Pharmacodynamic
Pharmacotherapeutic Group – Antivirals, nucleoside, nucleotide, reverse transcriptase inhibitors
Mechanism of action
Lamivudine is a nucleoside analogue which has activity against human immunodeficiency virus (HIV) and hepatitis B virus (HBV). It is metabolized intracellularly to the active moiety, lamivudine 5*triphosphate. Its main mode of action is as a chain terminator of viral reverse transcription. The triphosphate has selective inhibitory activity against HIV-1 and HIV-2 replication in vitro. The Lamivudine triphosphate acts as a substrate for HBV viral polymerase.
Lamivudine triphosphate does not interfere with normal cellular deoxynucleotide metabolism.
Pharmacokinetics
Absorption
Lamivudine is well absorbed from the gastrointestinal tract, and the bioavailability of oral lamivudine in adults is normally between 80 and 85%. Following oral administration, the mean time (t max) to maximal serum concentrations (C max) is about an hour. At therapeutic dose levels i.e. 100 mg once daily, Cmax is in the order of 1.1-1.5 μg/mL and trough levels were 0.015-0.020 μg/mL.
Co-administration of lamivudine with food resulted in a delay of tmax and a lower Cmax (decreased by up to 47%). However, the extent (based on the AUC) of lamivudine absorbed was not influenced, therefore lamivudine can be administered with or without food.
Distribution
From intravenous studies reported the mean volume of distribution is 1.3 l/kg. Lamivudine exhibits linear pharmacokinetics over the therapeutic dose range and displays low plasma protein binding to albumin.
Limited data shows lamivudine penetrates the central nervous system and reaches the cerebrospinal fluid (CSF). The mean lamivudine CSF/serum concentration ratio is 2-4 hours after oral administration was approximately 0.12.
Biotransformation
Lamivudine is predominately cleared by renal excretion in unchanged form. The likelihood of metabolic substance Interactions with lamivudine is low due to the small (5-10%) extent of hepatic metabolism and the low plasma protein binding.
Elimination
The mean systemic clearance of lamivudine is approximately 0.3 l/h/kg. The observed half-life of elimination is 5 to 7 hours. The majority of lamivudine is excreted unchanged in the urine via glomerular filtration and active secretion (organic cationic transport system). Renal clearance accounts for about 70% of lamivudine elimination.
In special populations
Studies in patients with renal impairment show lamivudine elimination is affected by renal dysfunction. Dose reduction in patients with a creatinine clearance of < 50 mL/min is necessary.
The pharmacokinetics of lamivudine are unaffected by hepatic impairment. Limited data In patients undergoing liver transplantation, show that impairment of hepatic function does not impact significantly on the pharmacokinetics of lamivudine unless accompanied by renal dysfunction.
In elderly patients the pharmacokinetic profile of lamivudine suggests that normal aging with accompanying renal decline has no clinically significant effect on lamivudine exposure, except in patients with creatinine clearance of < 50 mL/min.
Indication
Lamitec 100 is indicated for the treatment of chronic hepatitis B in adults with:
• compensated liver disease with evidence of active viral replication, persistently elevated serum alanine aminotransferase (ALT) levels and histological evidence of active liver inflammation and/or fibrosis. Initiation of lamivudine treatment should only be considered when the use of an alternative antiviral agent with a higher genetic barrier is not available or appropriate.
• decompensated liver disease in combination with a second agent without cross-resistance to lamivudine
DOSAGE AND ADMINISTRATION:
Therapy with Lamivudine should be initiated by a physician experienced in the management of chronic hepatitis B.
Posology
Adults
The recommended dosage of Lamitec 100 is 1 tablet per day.
In patients with decompensated liver disease, lamivudine should always be used in combination with a second agent, without cross-resistance to lamivudine, to reduce the risk of resistance and to achieve rapid viral suppression.
Duration of treatment
The optimal duration of treatment is unknown.
• In patients with HBeAg-positive chronic hepatitis B (CHB) without cirrhosis, treatment should be administered for at least 6-12 months after HBeAg seroconversion (HBeAg and HBV DNA loss with HBeAb detection) Is confirmed, to limit the risk of virological relapse. or until HBAg seroconversion or there is loss of efficacy. Serum ALT and HBV DNA levels should be followed regularly after treatment discontinuation to detect any late virological relapse.
• In patients with HBeAg negative CHB (pre-core mutant) without cirrhosis, treatment should be administered at least until HBs seroconversion or there is evidence of loss of efficacy. With prolonged treatment, regular reassessment is recommended to confirm that continuation of the selected therapy remains appropriate for the patient.
• In patients with decompensated liver disease or cirrhosis and in liver transplant recipients, treatment cessation is not recommended.
If Lamitec 100 is discontinued, patients should be periodically monitored for evidence of recurrent hepatitis
In special populations
Renal impairment
Lamivudine serum concentrations (AUC) are increased in patients with moderate to severe renal impairment due to decreased renal clearance. The dosage should therefore be reduced for patients with a creatinine clearance of < 50 mL/minute. When doses below 100 mg are required lamivudine oral solution should be used.
Hepatic impairment
No dose adjustment is necessary in patients with hepatic impairment unless accompanied by renal impairment.
Elderly
In elderly patients, normal aging with accompanying renal decline has no clinically significant effect on lamivudine exposure, except in patients with creatinine clearance of < 50 mL/min.
Pediatric population
The safety and efficacy of Lamivudine tablets in infants, children and adolescents aged below 18 years have not been established.
Method of administration
Oral use.
Lamitec 100 can be taken with or without food.
CONTRAINDICATIONS
Hypersensitivity to the active substance or to any of the excipients listed in formulation.
WARNINGS AND PRECAUTIONS
Exacerbations of hepatitis
Exacerbations on treatment: Spontaneous exacerbations in chronic hepatitis B are relatively common and are characterized by transient increases in serum ALT. After initiating antiviral therapy, serum ALT may increase in some patients as serum HBV DNA levels decline. In patients with compensated liver disease, these Increases in serum ALT were generally not accompanied by an increase in serum bilirubin concentrations or signs of hepatic decompensation.
Exacerbations after treatment discontinuation: Acute exacerbation of hepatitis has been observed in patients who have discontinued hepatitis B therapy and is usually detected by serum ALT elevations and re-emergence of HBV DNA. For lamivudine-treated patients, the majority of post treatment ALT elevations occurred between 8 and 12 weeks post-treatment.
Most events have been self-limiting, however some fatalities have been observed. If Lamivudine Tablets is discontinued, patients should be periodically monitored both clinically and by assessment of serum liver function tests (ALT and bilirubin levels), for at least four months, and then as clinically indicated.
Exacerbations in patients with decompensated cirrhosis: Transplantation recipients and patients with decompensated cirrhosis are at greater risk from active viral replication. Due to the marginal liver function in these patients, hepatitis reactivation at discontinuation of lamivudine or loss of efficacy during treatment may induce severe and even fatal decompensation. These patients should be monitored for clinical, virological and serological parameters associated with hepatitis B, liver and renal function, and antiviral response during treatment (at least every month), and, if treatment is discontinued for any reason, for at least 6 months after treatment. Laboratory parameters to be monitored should include (as a minimum) serum ALT, bilirubin, albumin, blood urea nitrogen, creatinine, and virological status: HBV antigen/antibody, and serum HBV DNA concentrations when possible. Patients experiencing signs of hepatic insufficiency during or post-treatment should be monitored more frequently as appropriate.
For patients who develop evidence of recurrent hepatitis post-treatment, there are Insufficient data on the benefits of re-initiation of lamivudine treatment.
Mitochondrial dysfunction
Nucleoside and nucleotide analogues have been demonstrated in vitro and in vivo to cause
a variable degree of mitochondrial damage. There have been reports of mitochondrial dysfunction in Infants exposed in utero and/or post-natally to nucleoside analogues. The main adverse events reported are haematological disorders (anaemia, neutropenia), metabolic disorders (hyperlipasemia). Some late-onset neurological disorders have been reported (hypertonia, convulsion, abnormal behaviour). The neurological disorders might be transient or permanent. Any chiid exposed in utero to nucleoside and nucleotide analogues, should have clinical and laboratory follow-up and should be fully investigated for possible mitochondrial dysfunction in cases which have relevant signs or symptoms.
Pediatric patients
Lamivudine has been administered to children (2 years and above) and adolescents with compensated chronic hepatitis B. However, due to limitations of the data, the administration of lamivudine to this patient population is not currently recommended.
Delta hepatitis or hepatitis C
The efficacy of lamivudine in patients co-infected with Delta hepatitis or hepatitis C has not been established and caution is advised.
Immunosuppressive treatments
Data are limited on the use of lamivudine in HBeAg-negative (pre-core mutant) patients and in those receiving concurrent immunosuppressive regimes, including cancer chemotherapy.
Lamivudine should be used with caution in these patients.
Monitoring
During treatment with Lamivudine Tablets patients should be monitored regularly. Serum ALT and HBV DNA levels should be monitored at 3 month intervals and in HBeAg-positive patients HBeAg should be assessed every 6 months.
HIV co-infection
For the treatment of patients who are co-infected with HIV and are currently receiving or plan to receive treatment with lamivudine or the combination lamivudine-zidovudine, the dose of lamivudine prescribed for HIV infection (usually 150 mg/twice daily in combination with other antiretrovirals) should be maintained. For HIV co-infected patients not requiring anti-retroviral therapy, there is a risk of HIV mutation when using lamivudine alone for treating chronic hepatitis B.
Transmission of hepatitis B
There Is limited information available on maternal-fetal transmission of hepatitis B virus in pregnant women receiving treatment with lamivudine. The standard recommended procedures for hepatitis B virus immunization in infants should be followed.
Patents should be advised that therapy with lamivudine has not been proven to reduce the risk of transmission of hepatitis B virus to others and therefore, appropriate precautions should still be taken.
Interactions with other medicinal products
Lamivudine should not be taken with any other medicinal products containing lamivudine or medicinal products containing emtricitabine.
The combination of lamivudine with cladribine is not recommended.
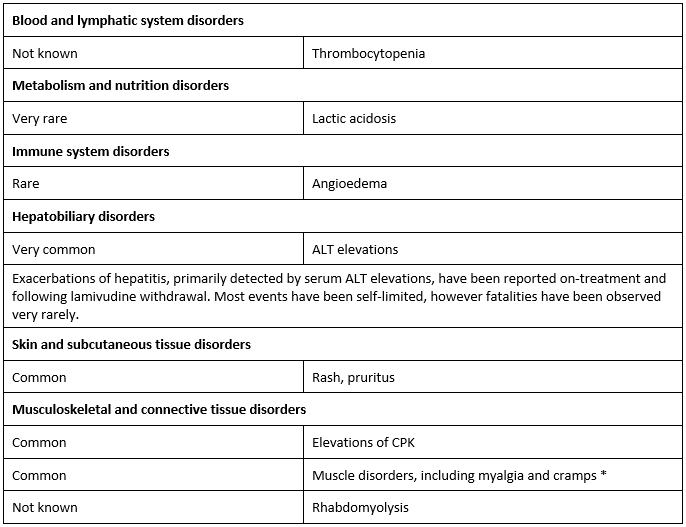
Undesirable effects
The most common adverse effects are fatigue, respiratory tract infection, throat and tonsil discomfort, headache, abdominal discomfort and pain, nausea, vomiting and diarrhoea.
• In Phase III studies frequency observed in the lamivudine treatment group was not greater than observed in the placebo group.
Paediatric population
Based on limited data in children aged 2 to 17 years, there were no new safety Issues identifled
compared to adults.
Other special populations
In patients with HIV Infection, cases of pancreatitis and peripheral neuropathy (or paraesthesia) have been reported. In patients with chronic hepatitis B there was no observed difference In Incidence of these events between placebo and lamivudine-treated patients.
DRUG INTERACTIONS
The likelihood of metabolic interactions is low due to limited metabolism and plasma protein binding and almost complete renal elimination of unchanged substances.
Administration of trimethoprim/sulfamethoxazole 160 mg/800 mg increased lamivudine exposure by about 40%. Lamivudine had no effect on the pharmacokinetics of trimethoprim or sulfamethoxazole. However, unless the patient has renal impairment, no dosage adjustment of lamivudine is necessary.
A modest increase in max (28 %) was observed for zidovudine when administered with lamivudine, however overall exposure (AUC) was not significantly altered. Zidovudine had no effect on the pharmacokinetics of lamivudine.
Lamivudine has no pharmacokinetic interaction with alpha-interferon when the two medicinal products are concurrently administered.
The concomitant use of lamivudine with cladribine is not recommended.
Emtricitabine
Due to similarities, Lamivudine should not be administered concomitantly with other cytidine analogues, such as emtricitabine. Moreover, Lamivudine should not be taken with any other medicinal products containing lamivudine.
Use in Specific Populations
Pregnancy
Animal studies with lamivudine showed an increase in early embryonic deaths in rabbits but not in rats. Placental transfer of lamivudine has been shown to occur in humans.
Lamivudine can be used during pregnancy if clinically needed.
For patients who are being treated with lamivudine and subsequently become pregnant consideration should be given to the possibility of a recurrence of hepatitis on discontinuation of lamivudine.
Breast-feeding
Breast-feeding mothers being treated with lamivudine for HBV taking into account the benefit of breastfeeding for the child and the benefit of therapy for the woman. Where there Is maternal transmission of HBV, despite adequate prophylaxis, consideration should be given to discontinuing breastfeeding to reduce the risk of the emergence of lamivudine-resistant mutants in the infant.
Fertility
Reproductive studies in animals have shown no effect on male or female fertility.
OVERDOSAGE
Administration of lamivudine at very high dose levels in acute animal studies did not result In any organ toxicity. Limited data are available on the consequences of ingestion of acute overdoses in humans. No fatalities occurred, and the patients recovered. No specific signs or symptoms have been identified following such overdose.
If overdose occurs the patient should be monitored and standard supportive treatment applied as required. Since lamivudine is dialysable, continuous hemodialysis could be used In the treatment of overdose, although this has not been studied.
Presentation:
Alu-Alu Pack of 3*10 Tablets
Storage
Sorte below 30 degree C. Protect form light and moisture.
Shelf Life: 36 Months
Myanmar Regn No. : R2403A6702
Manufactured by:
Sai Mirra Innopharm Pvt. Ltd.
288 and 299, SIDCO Estate, Chennai 600 098, India
Product of:
Zifam Pinnacle Pty Ltd
Sydney, Australia