Zifam Tenovir
- ENG
- မြန်မာ
Composition:
Each film coated tablet contains:
Tenofovir Disoproxil Fumarate 300 mg
(To Provide Tenafovir Disoproxil 245 mg)
Excipients: q.s
Colour: Tartrazine Yellow.
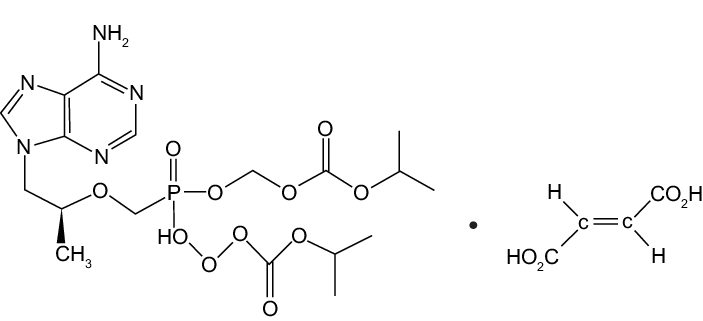
Mollecular Structure:
ZIFAM TENOVIR is the brand name for tenofovir disoproxil fumarate (a prodrug of tenofovir) which is a fumaric acid salt of bis-isopropoxycarbonyloxymethyl ester derivative of tenofovir. In vivo tenofovir disoproxil fumarate is converted to tenofovir, an acyclic nucleoside phosphonate (nucleotide) analog ofadenosine 5′-monophosphate. Tenofovir exhibits activity against HIV-1reverse transcriptase.
The chemical name of tenofovir disoproxil fumarate is 9-[(R)-2[[bis[[(Isopropoxycarbonyl)oxy]methoxy]phosphinyl|methoxy|pr opyl]adenine fumarate (1:1). It has a molecular formula of CH,N.O,P C,H,O, and a molecular weight of 635.52. It has the following structural formula:
Dosage & Administration:
Recommended dose for the treatment of HIV-1 or chronic hepatitis B in adults and pediatric patients 12 years of age and older (35 kg or more): 300 mg once daily taken orally without regard to food.
Recommended dose for the treatment of HIV-1 in pediatric patients (2 to less than 12 years of age):
Tablets: for pediatric patients weighing greater than or equal to 17 kg who can swallow an intact tablet, one ZIFAM TENOVIR tablet (half or full 300 mg based on body weight) once daily taken orally without regard to food.
Dose recommended in renal impairment in adults:
Creatinine clearance 30-49 mL/min: 300 mg every 48 hours.
Creatinine clearance 10-29 mL/min: 300 mg every 72 to 96 hours.
Hemodialysis: 300 mg every 7 days or after approximately 12 hours of dialysis.
Dosage Forms & Strengths:
Each Film-coated tablet contains: 300 mg Tenofovir Disoproxil Fumarate.
Pharmacokinetics:
The pharmacokinetics of tenofovir disoproxil fumarate have been evaluated in healthy volunteers and HIV-1 infected individuals. Tenofovir pharmacokinetics are similar between these populations
Absorption:
ZIFAM TENOVIR is a water-soluble diester prodrug of the active ingredient tenofovir. The oral bioavailability of tenofovir from ZIFAM TENOVIR in fasted patients is approximately 25%. Following oral administration of a single dose of ZIFAM TENOVIR 300 mg to HIV-1 infected patients in the fasted state, maximum serum concentrations (Cmax) are achieved in 1.0 ‡ 0.4 hrs. Cmax and AUC values are 0.30 ‡ 0.09 pg/mL and 2.29 ‡ 0.69 pg hr/mL, respectively.
The pharmacokinetics of tenofovir are dose proportional over a ZIFAM TENOVIR dose range of 75 to 600 mg and are not affected by repeated dosing.
Distribution:
In vitro binding of tenofovir to human plasma or serum proteins is less than 0.7 and 7.2%, respectively, over the tenofovir concentration range 0.01 10 25 ugimL. The volume of distribution at steady-state is 1.3: 0.6 L/kg and 12 ‡ 04 L/kg, following Intravenous administration of tenofovir 1.0mg/kg and 3.0 mg/kg respectively.
Metabolism and Elimination:
In vitro studies indicate that neither tenotovir disoprox nor tenofovir are substrates of CYP enzymes.
Following IV administration of tenofovir, approximately 70-30% of the dose is recovered in the urine as unchanged tenofovir within 72 hours of dosing. Fallowing single dose, oral administration of ZIFAM TENOVIR, the terminal elimination half-life of tenofovir is approximately 17 hours. After multiple oral doses of ZIFAM TENOVIR 300 mg once daily (under fed conditions), 32 ‡ 10% of the administered dose is recovered in urine over 24 hours.
Tenofovir is eliminated by a combination of glomerular filtration and active tubular secretion. There may be competition for elimination with other compounds that are also renally eliminated.
Warnings & Precaution:
- New onset or worsening renal impairment: Can include acute renal failure and Fanconi syndrome. Assess creatinine clearance (CrCI) before initiating treatment with ZIFAM TENOVIR. Monitor CrCl and serum phosphorus in patients at risk. Avoid administering ZIFAM TENOVIR with concurrent or recent use of nephrotoxic drugs.
- Coadministration with Other Products: Do not use with other tenofovir-containing products – Do not administer in combination with adefovir dipivoxil.
- Decreases in bone mineral density (BMD): Consider assessment of BMD in patients with a history of pathologic fracture or other risk factors for osteoporosis or bone loss.
- Redistribution/accumulation of body fat: Observed in HIV-infected patients receiving antiretroviral combination therapy.
- Immune reconstitution syndrome: Observed in HIV-infected patients. May necessitate further evaluation and treatment.
- Triple nucleoside-only regimens: Early virologic failure has been reported in HIV-infected patients. Monitor carefully and consider treatment modification.
- HIV testing: HIV antibody testing should be offered to all HBV Infected patients before initiating therapy with ZIFAM TENOVIR. ZIFAM TENOVIR should only be used as part of an appropriate antiretroviral combination regimen in HIV-Infected patients with or without HBV confection.
Adverse Reactions:
In HIV-infected adult subjects: Most common adverse reactions (Incidence greater than or equal to 10%, Grades 2 4) are rash, diarrhea, headache, pain, depression, asthenia, and nausea. In HEV-infected subjects with compensated liver disease: most common adverse reaction (all grades) was nausea (9%).
In pediatric subjects: Adverse reactions in pediatric subjects were consistent with those observed in adults.
In HBV-infected subjects with decompensated liver disease: most common adverse reactions (incidence greater than or equal to 10%, all grades) were abdominal pain, nausea, insomnia, prunitus, vomiting, dizziness, and pyrexia.
Drug Interactions:
- Didanosine: Coadministration increases didanosine concentrations. Use with caution and monitor for evidence of didanosine toxicity (e.g., pancreatitis, neuropathy). Consider dose reductions or discontinuations of didanosine if warranted.
- Alazanavir: Coadministration decreases atazanavir concentrations and increases tenofovir concentrations. Use atazanavir with ZIFAM TENOVIR only with additional ritonavir, monitor for evidence of tenofovir toxicity.
- Lopinavir/ritonavir: Coadministration increases tenofovir concentrations. Monitor for evidence of tenofovir toxicity.
Dosage:
As directed by the Physician.
Storage:
Store in a cool and dry place below 30°C. Protect from light and moisture, Keep Medicine away from children.
Presentation:
Blister pack of 10 tablets.
Related Products